|
The cytoplasm of living cells is separated from the environment by means of membranes. A complete shutoff from the surroundings however is not useful: there has to be some exchange of materials to enable metabolism. To accomplish this there have to be defined pores in the membranes.
In microorganisms the regulation of the exchange of metabolites depends on the very near surroundings of the cell. In multicellular organisms there is a transport problem between different organs over longer distances, too. In animals the energy supply substance glucose has to be excreted by one kind of cells into the circulatory system, other cells have to import it, both in well tuned regulation. Plants have to transport sucrose and amino acids between different tissues. Transport of substances across membranes often requires energy to overcome a concentration gradient. The water balance of higher organisms is actively regulated by the aquaporins, shifting in man in excess of 150 liters per day.
In many organisms the genetics of proteins enabling diffusion or transport of all kind of substances through membranes are well understood. The determination of molecular structures at atomic resolution is somewhat more difficult as few membrane proteins (or by engineering modifyed ones) are able to be crystallized. Some data were obtained by nuclear magnetic resonance investigations of solubilized proteins. In the following some examples of (mostly bacterial) structures are shown.
A classification of these proteins may follow different rules: by function the proteins may guide diffusion or facilitated diffusion or active transport against a concentration gradient of small molecules. For active transport there has to be some coupling to an energy delivering process (e.g. a countercurrent transport). Another rule is typing by the substance moved, e.g. glucose transporter. Physical properties of transport systems allow a distiction of trimeric porins from monomeric channels or binding protein type systems. Another classification discerns systems with unchanged solutes from those, in which the transported substance is chemically modified as in the phosphoenol pyruvate:sugar phosphotransferase system. Depending on the genetics transport proteins displaying sequence homology may be grouped into families irrespective of their transport tasks.
|
|
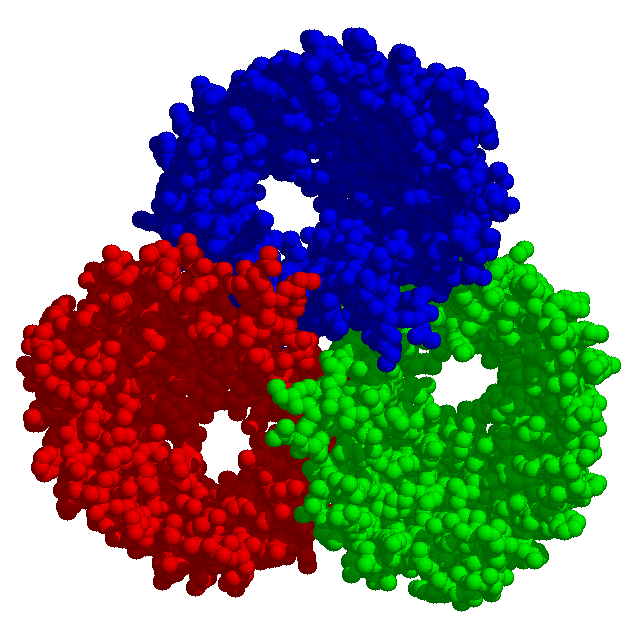 The permeability of a membrane may be of rather adverse advantage to a cell. Toxins liberate some cellular contents which are consumed by the toxin producer, thereby damaging the punctured target cell. On the other hand the antibiotic properties of such substances may be of therapeutic value.
The permeability of a membrane may be of rather adverse advantage to a cell. Toxins liberate some cellular contents which are consumed by the toxin producer, thereby damaging the punctured target cell. On the other hand the antibiotic properties of such substances may be of therapeutic value.
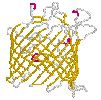
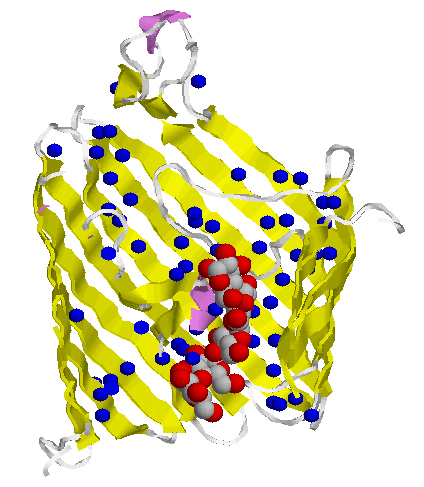
Bacterial toxins (to the left rotates α-hemolysin from Staphylococcus aureus) and porins differ from other membrane proteins by their construction principle. The channels formed by them are enabled by barrels made from beta-strands. They got large diameters and lack specificity regarding the solutes to be passed. Porins are to be found in mitochondria and chloroplasts, too. In bacteria with two membrane envelopes and intervening periplasmic space the porins are located in the outer membrane. Specific transport systems and active ones are to be found in the inner cytoplasmic membrane. They are predominantly helical in structure.
|
(cut away view) from Escherichia coli |
Literature:
D Rentsch et al, Structure and function of plasma membrane amino acid, oligopeptide and sucrose transporters from higher plants, J. Membrane Biol. 162 (1998) 177-190
P Agre et al, The aqua porins - blueprints for cellular plumbing systems, J. Biol. Chem. 273 (1998) 14659-14662
T Schirmer, General and specific porins from bacterial outer membranes, J. Struct. Biol. 121 (1998) 101-109

