Herbicides against toxoplasmosis
The etiological agent of toxoplasmosis is the protozoan parasite Toxoplasma gondii. The parasite is characterized by an essential nonphotosynthetic plastid-like organelle. Any metabolism in such organelles if specific to these compartments may be the target of inhibitors which exert their antibiotic action only on the parasite without affecting the host.
Fatty acid biosynthesis is essential to all cellular organisms. The rate-determining step in this process is the addition of carbon dioxide to acetyl-CoA to generate malonyl-CoA which is catalyzed by acetyl-CoA carboxylase (ACC). In plants these enzymes are located in chloroplasts. There are two types of ACC: in monocotyledous plants the enzyme consists of a large multidomain protein, whereas dicotyledous plants and other organisms (including bacteria and man) harbour a multisubunit version. A prominent functional difference between these types of enzymes is their sensitivity against herbicides. The single chain proteins are sensitive to aryloxyphenoxypropionates (fops) and cyclohexanediones (dims), whereas the multisubunit enzymes are resistant. It turned out that Toxoplasma gondii contains ACCs of the multidomain version of the enzyme.
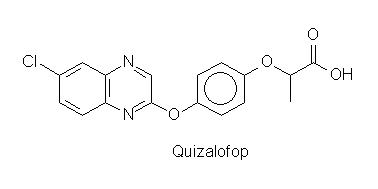
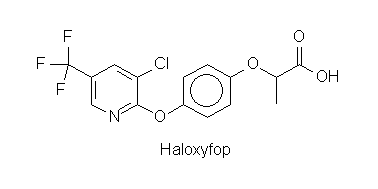
When the activity of ACC from T. gondii was tested for inhibition by fops or dims a strong effect was found for three of four fops used in agriculture but for none of the dims. The same result was obtained for incorporation of labelled uracil into cells of T. gondii. As control the incorporation of tymidine into human fibroblasts was measured with the same concentrations of herbicides. Fibroblast growth was not affected, when parasite growth was stalled. Fop-type herbicides thus may find an important future medical use in treatment of toxoplasmosis (and diseases caused by parasites with similar physiology).
 |  |
E Zuther et al., Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase, Proc. Natl. Acad. Sci. USA 96 (1999) 13387-13392
About T. gondii: http://www.sas.upenn.edu/~striepen/
1-2000 © Rolf Bergmann